Registrations and Approvals
your reliable regulatory partner, ensuring products follow the regulations throughout their life cycle.

Pharmaceuticals Regulatory Services
We provide end-to-end regulatory services: from initial assessment, dossier preparation and submission to approval as well as the entire range of post-marketing services that are required.

Nutraceuticals Regulatory Services
With ever continues changes and updates to regulatory matters related to Libya’s FDCC compliance and import of Nutraceuticals and Food Supplements, all related assistance is handled by us with strategic planning and technical acumen.
Medical Devices Regulatory Services
With advancing medical technology and the exponentially rising need of medical devices in the country, new reforms and rules have come into effect
At Almasa Aldwaeia, we help manufacturers overcome the barriers in preparing strong regulatory strategies, we provide end-to-end regulatory services: from initial assessment, dossier preparation and submission to approval as well as the entire range of post-marketing services that are required.
ou can benefit from our broad technical registration expertise and in-depth knowledge of local regulations.
We are your single point of contact for all regulatory matters, as well as commercial and distribution services.
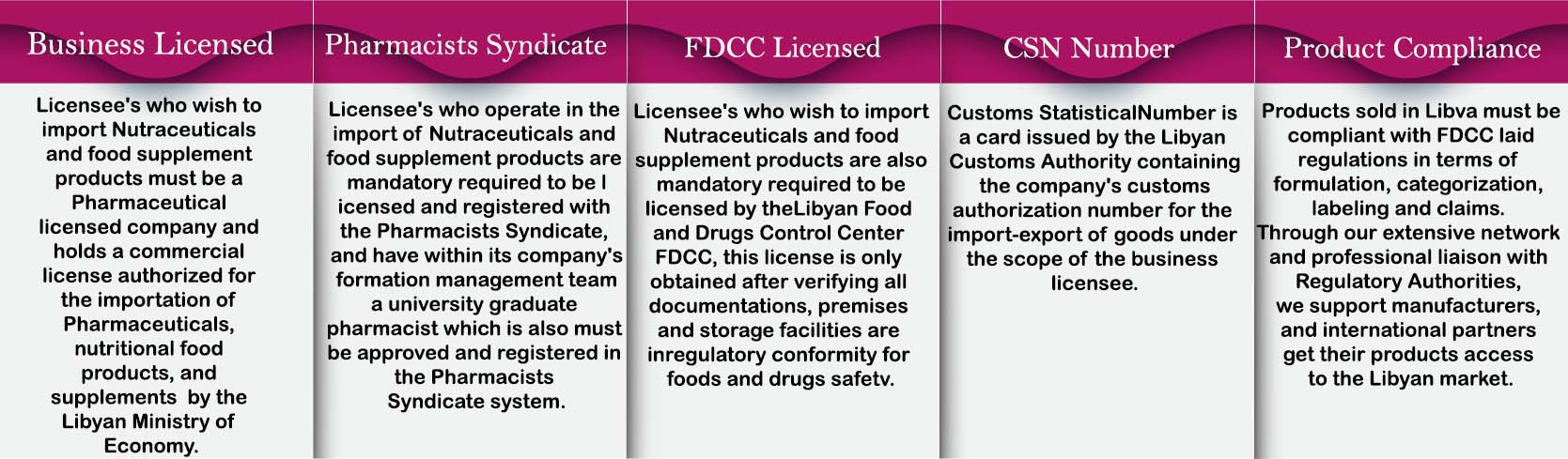
LIBYAN MARKET ENTRY
( LMOH Drug Registration Requirements )
Reliable regulatory partner, ensuring products follow the regulations throughout their life cycle.
Almasa Aldwaeia representative can assist you by providing the exact information you need to make an informed decision. Please Contact us.